Sensipar® plus vitamin D and phosphate binders*
lowered PTH, phosphorus, and calcium2
PHASE 3 POOLED STUDIES
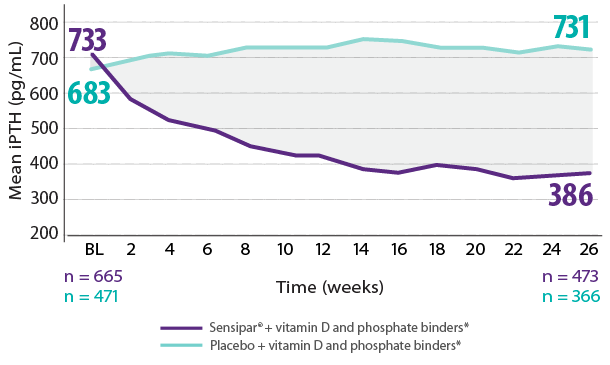
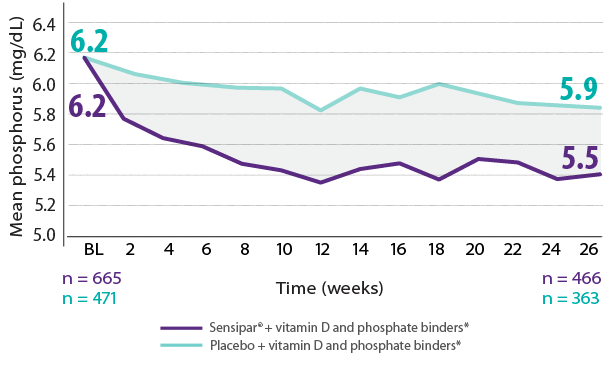
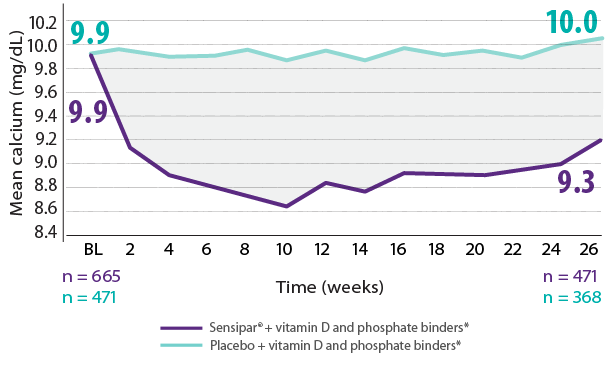
Results are pooled from a retrospective analysis of three phase 3, 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies comparing Sensipar® with placebo in patients with CKD on dialysis with iPTH ≥ 300 pg/mL and serum calcium ≥ 8.4 mg/dL (N = 1,136). Patients in both treatment arms could be treated with vitamin D sterols and/or phosphate binders. Mean baseline iPTH values in the Sensipar® group and placebo group were 733 pg/mL and 683 pg/mL, respectively. The primary endpoint of the study was the proportion of patients achieving PTH level ≤ 250 pg/mL during the efficacy assessment phase (EAP).1,2
*Vitamin D and/or phosphate binders. Not all patients received vitamin D or phosphate binders.
KDIGO = Kidney Disease Improving Global Outcomes; sHPT = secondary hyperparathyroidism.
- In phase 3 trials, 40% of Sensipar® patients achieved iPTH ≤ 250 pg/mL vs 5% with placebo (P < 0.001)1
- Median doses of vitamin D and phosphate binders were similar at baseline and at end of study2
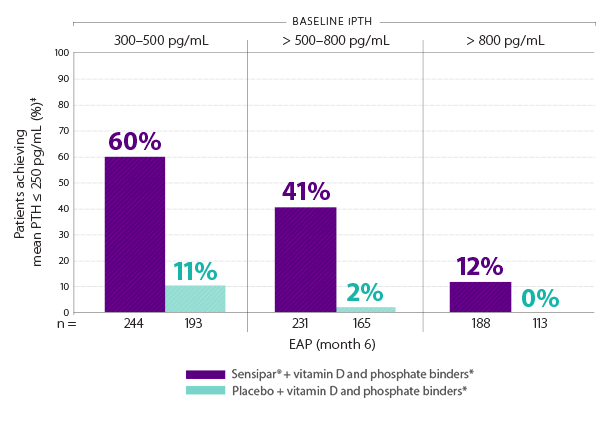
Initiating Sensipar® with vitamin D and phosphate binders* at iPTH 300–500 pg/mL helped 60% of patients achieve study PTH treatment goal1,16†
- In phase 3 trials, 40% of Sensipar® patients achieved iPTH ≤ 250 pg/mL vs 5% with placebo (P < 0.001)1
Patients with milder disease typically require lower Sensipar® doses1
- The mean dose of study drug during the efficacy assessment phase for subjects who achieved iPTH ≤ 250 pg/mL was 85 mg16
- In phase 3 studies, the Sensipar® dose was increased unless one of the following applied: iPTH ≤ 200 pg/mL, serum calcium < 7.8 mg/dL, or the subject was experiencing an adverse event that precluded a dose increase16
†Treatment goal was defined as mean iPTH ≤ 250 pg/mL.1
‡Intent-to-treat analysis population.
EAP = efficacy assessment phase.
Initiating Sensipar® with vitamin D and phosphate binders* at iPTH 300–500 pg/mL helped 40% of patients achieve PTH ≤ 300 pg/mL and phosphorus 3.5–5.5 mg/dL17
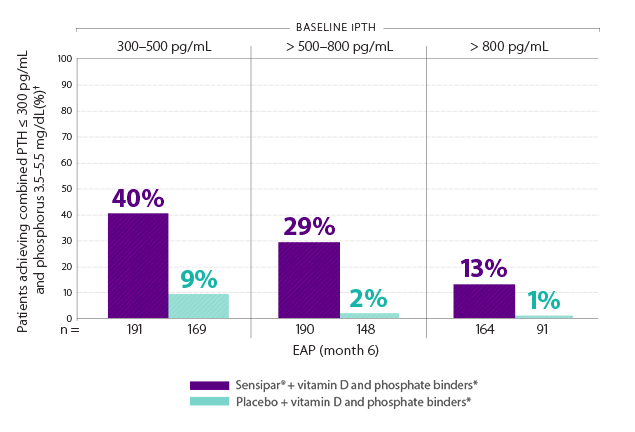
- In phase 3 trials, 40% of Sensipar® patients achieved iPTH ≤ 250 pg/mL vs 5% with placebo (P < 0.001)1
†Intent-to-treat analysis population.
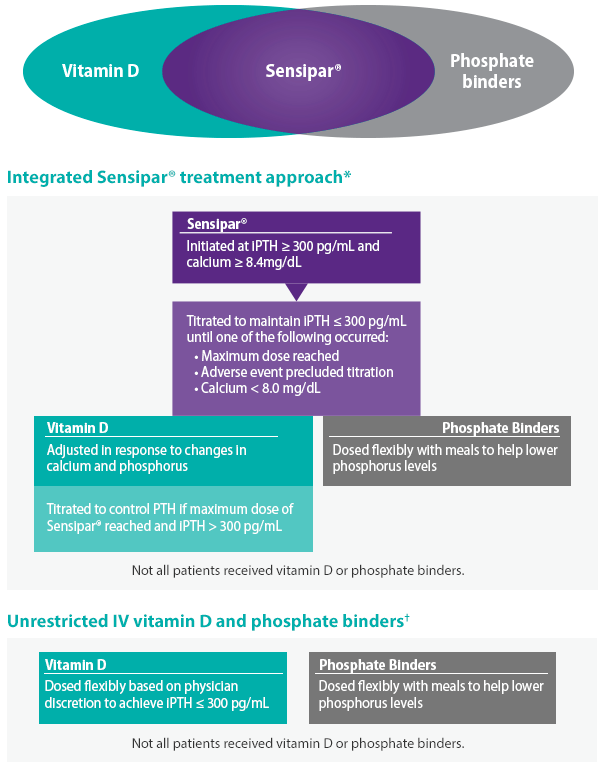
Sensipar® plus vitamin D and phosphate binders*: an integrated approach to secondary HPT treatment in patients on dialysis11-13
Sensipar® plus vitamin D and phosphate binders* lowered PTH, phosphorus, and calcium11
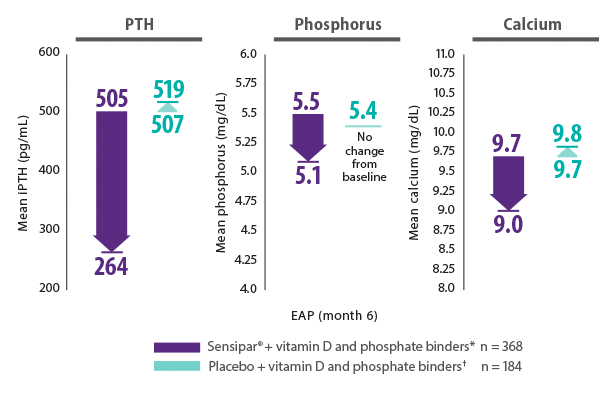
- In the OPTIMA study, mean dialysis vintage in the Sensipar® treatment group and unrestricted vitamin D and phosphate binders group were 64.1 and 69.4 months, respectively11
- In phase 3 trials, 40% of Sensipar® patients achieved iPTH ≤ 250 pg/mL vs 5% with placebo (P < 0.001)1
Sensipar® plus vitamin D and phosphate binders* lowered PTH phosphorus and calcium12
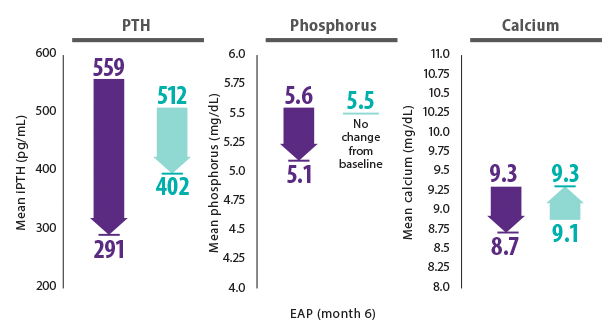
- In the Incident Dialysis Study, patients were new to dialysis (3–12 months). Mean time on dialysis was 7.2 months12
- In phase 3 trials, 40% of Sensipar® patients achieved iPTH ≤ 250 pg/mL vs 5% with placebo (P < 0.001)1
Managing calcium in patients taking Sensipar® (cinacalcet)1

|
Sensipar® treatment should not be initiated if serum calcium is less than the lower limit of the normal range (8.4 mg/dL). Serum calcium should be measured within 1 week after initiation or dose adjustment of Sensipar®. Once the maintenance dose has been established, serum calcium should be measured approximately monthly. |

|
If serum calcium falls below 8.4 mg/dL but remains above 7.5 mg/dL, or if symptoms of hypocalcemia occur, calcium-containing phosphate binders and/or vitamin D sterols can be used to raise serum calcium. |

|
If serum calcium falls below 7.5 mg/dL, or if symptoms of hypocalcemia persist and the vitamin D dose cannot be increased, withhold administration of Sensipar® until serum calcium reaches 8.0 mg/dL and/or symptoms of hypocalcemia have resolved. Treatment should be reinitiated using the next lowest dose of Sensipar®. |
- Significant lowering of serum calcium can cause paresthesias, myalgias, muscle spasms, tetany, seizures, QT interval prolongation, and ventricular arrhythmia
CALCIUM LEVELS
Serum calcium reductions with Sensipar® were lower among patients initiated at the lowest baseline calcium levels (8.4–9.5 mg/dL)14*
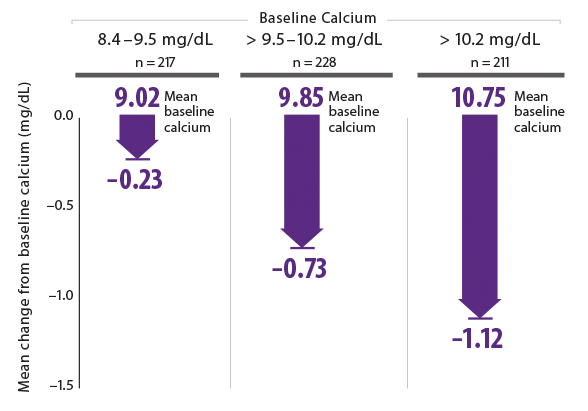
*Sensipar®-treated patients only.
After initiation of Sensipar®, patients who maintained calcium ≥ 8.4 mg/dL had the lowest baseline serum PTH levels15
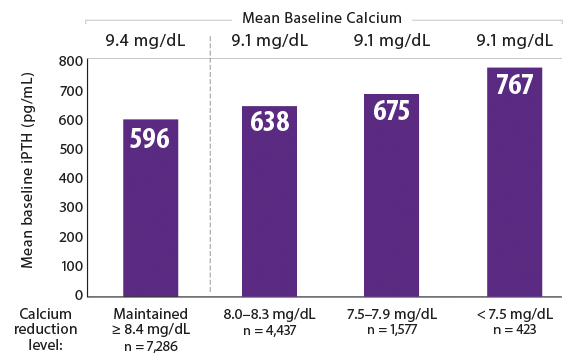
- Median Sensipar® dose was 30 mg per day across all cohorts1
- In 26-week studies of patients with secondary HPT and CKD on dialysis, 66% of patients receiving Sensipar® compared with 25% of patients receiving placebo developed at least one serum calcium value less than 8.4 mg/dL, whereas 29% of patients receiving Sensipar® compared with 11% of patients receiving placebo developed at least one serum calcium value less than 7.5 mg/dL. Less than 1% of patients in each group permanently discontinued study drug due to hypocalcemia1
Reductions in calcium with Sensipar® were most prominent in the first week of therapy but stabilized over time12
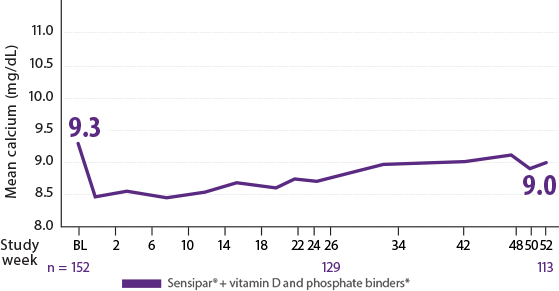
- Sixteen percent (16%) of patients receiving Sensipar® compared with 1% of patients receiving placebo developed an adverse event of hypocalcemia12

