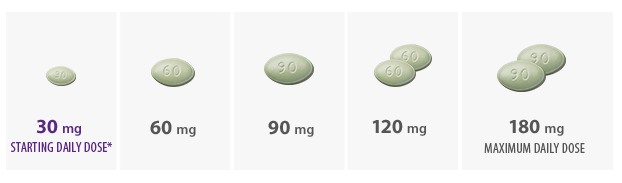
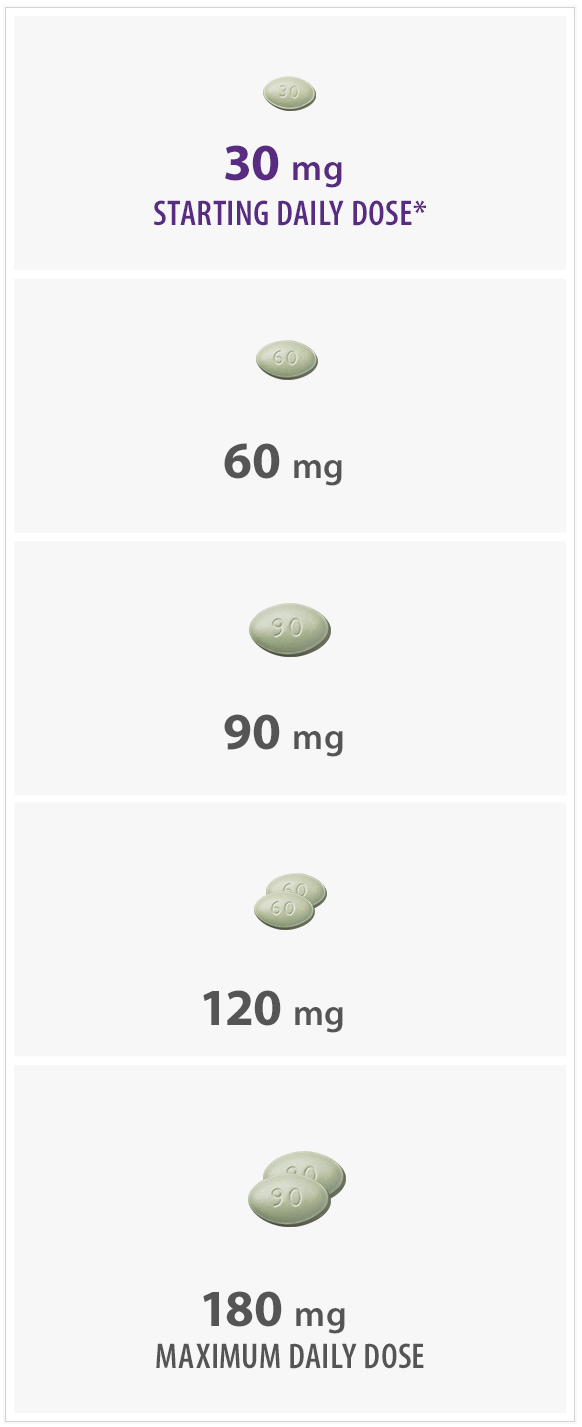
- Initiate Sensipar® at 30 mg once daily in patients with secondary HPT on dialysis with iPTH > 300 pg/mL and calcium ≥ 8.4 mg/dL
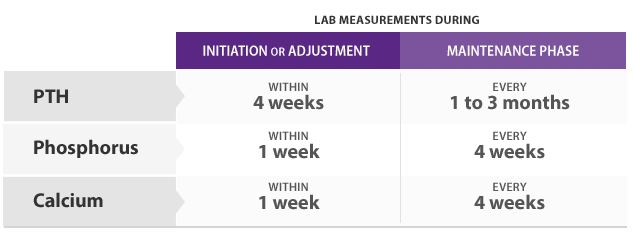
- PTH levels should be measured at least 12 hours post dose
- Constant serum drug levels (steady-state) are achieved within 7 days
TITRATION SCHEDULE
Pills not shown at actual size.
*The recommended starting dose is 30 mg.
HPT = hyperparathyroidism; iPTH = intact parathyroid hormone; PTH = parathyroid hormone.
*The recommended starting dose is 30 mg.
HPT = hyperparathyroidism; iPTH = intact parathyroid hormone; PTH = parathyroid hormone.
- Titrate no more frequently than every 2 to 4 weeks through sequential doses
- Sensipar® should be taken with food or shortly after a meal
- Sensipar® tablets are administered orally
- Sensipar® should always be taken whole, not chewed, crushed, or divided
The most commonly reported adverse reactions with Sensipar® (cinacalcet) were nausea, vomiting, and diarrhea1
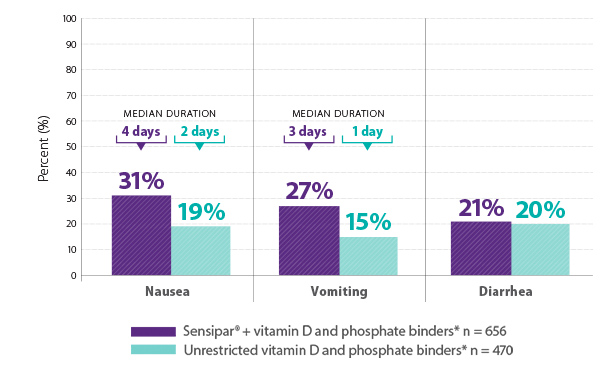
Results are pooled from a retrospective analysis of three phase 3, 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies comparing Sensipar® with placebo in patients with CKD on dialysis with iPTH ≥ 300 pg/mL and serum calcium ≥ 8.4 mg/dL (N = 1,136). Patients in both treatment arms could be treated with vitamin D sterols and/or phosphate binders. Mean baseline iPTH values in the Sensipar® group and placebo group were 733 pg/mL and 683 pg/mL, respectively. The primary endpoint of the study was the proportion of patients achieving PTH level ≤ 250 pg/mL during the efficacy assessment phase.1,2
- The mean duration of nausea in days was 18 and 22 for placebo and cinacalcet, respectively16
- The mean duration of vomiting in days was 11 and 16 for placebo and cinacalcet, respectively16
*Vitamin D and/or phosphate binders, if prescribed. Not all patients received vitamin D or phosphate binders.
PHASE 3 SAFETY SUBJECTS: INCIDENCE OF NAUSEA BY ACTION TAKEN16
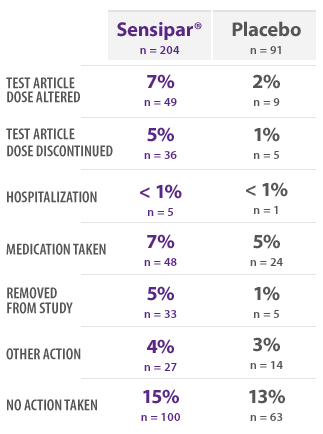
- This table provides information regarding actions taken in response to reports of nausea16
- Incidence of nausea was not dose dependent16
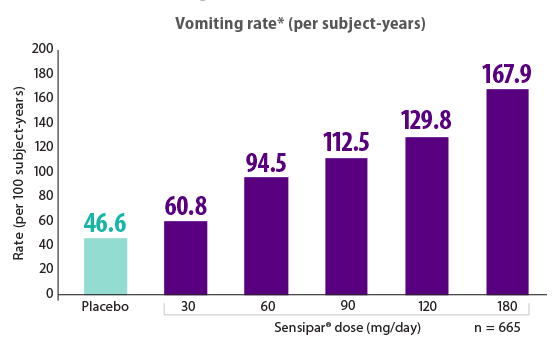
Lower doses of Sensipar® (cinacalcet) were associated with a lower incidence of vomiting16
Results are pooled from a retrospective analysis of three phase 3, 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies comparing Sensipar® with placebo in patients with CKD on dialysis with iPTH ≥ 300 pg/mL and serum calcium ≥ 8.4 mg/dL (N = 1,136). Patients in both treatment arms could be treated with vitamin D sterols and/or phosphate binders. Mean baseline iPTH values in the Sensipar® group and placebo group were 733 pg/mL and 683 pg/mL, respectively. The primary endpoint of the study was the proportion of patients achieving PTH level ≤ 250 pg/mL during the efficacy assessment phase.1,2
- There was no dose-response for nausea in cinacalcet-treated patients, but there was a clear dose-response for vomiting, as shown in the figure16
*Rate of vomiting = total circumstances of vomiting divided by the total exposure time (per 100 subject-years) over all subjects at that dose level.
iPTH = intact parathyroid hormone.
iPTH = intact parathyroid hormone.
PHASE 3 SAFETY SUBJECTS: INCIDENCE OF VOMITING BY ACTION TAKEN16
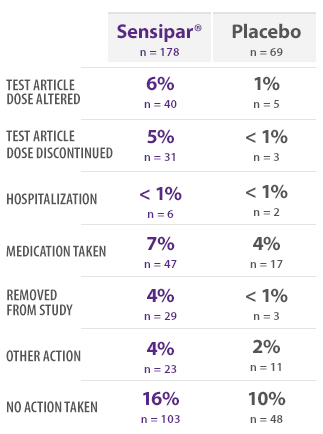
- This table provides information regarding actions taken in response to reports of vomiting16

