Contraindication: Sensipar® (cinacalcet) treatment initiation is contraindicated if serum calcium is less than the lower limit of the normal range (8.4 mg/dL).
Hypocalcemia: Sensipar® lowers serum calcium and can lead to hypocalcemia. Life threatening events and fatal outcomes associated with hypocalcemia have been reported in patients treated with Sensipar®, including pediatric patients. The safety and effectiveness of Sensipar® have not been established in pediatric patients.
Decreases in serum calcium can prolong the QT interval, potentially resulting in ventricular arrhythmia. Cases of QT prolongation and ventricular arrhythmia have been reported in patients treated with Sensipar®. Patients with conditions that predispose to QT interval prolongation and ventricular arrhythmia may be at increased risk for QT interval prolongation and ventricular arrhythmias if they develop hypocalcemia due to Sensipar®. Closely monitor corrected serum calcium and QT interval in patients at risk receiving Sensipar®.
Significant reductions in calcium may lower the threshold for seizures. Monitor serum calcium levels in patients with seizure disorders on Sensipar®.
Concurrent administration of Sensipar® with calcium-lowering drugs including other calcimimetics could result in severe hypocalcemia. Parsabiv™ (etelcalcetide) and Sensipar® should not be given together. Closely monitor serum calcium in patients receiving Sensipar® and concomitant therapies known to lower serum calcium levels.
Patients with secondary HPT: Serum calcium and serum phosphorus should be measured within 1 week and PTH should be measured 1 to 4 weeks after initiation or dose adjustment of Sensipar®.
Once the maintenance dose has been established, serum calcium and serum phosphorus should be measured approximately monthly, and PTH every 1 to 3 months.
Patients with primary HPT or parathyroid carcinoma: Serum calcium should be measured within 1 week after initiation or dose adjustment of Sensipar®. Once maintenance dose levels have been established, serum calcium should be measured every 2 months.
Upper Gastrointestinal Bleeding: Cases of gastrointestinal (GI) bleeding, mostly upper GI bleeding, have occurred in patients using calcimimetics, including Sensipar®, from postmarketing and clinical trial sources. The exact cause of GI bleeding in these patients is unknown.
Patients with risk factors for upper GI bleeding, such as known gastritis, esophagitis, ulcers or severe vomiting, may be at increased risk for GI bleeding with Sensipar®. Monitor patients for worsening of common Sensipar® GI adverse reactions and for signs and symptoms of GI bleeding and ulcerations during Sensipar® therapy.
Hypotension, Worsening Heart Failure and/or Arrhythmias: In Sensipar® postmarketing use, isolated, idiosyncratic cases of hypotension, worsening heart failure, and/or arrhythmia were reported in patients with impaired cardiac function. The causal relationship to Sensipar® therapy could not be completely excluded and may be mediated by reductions in serum calcium levels.
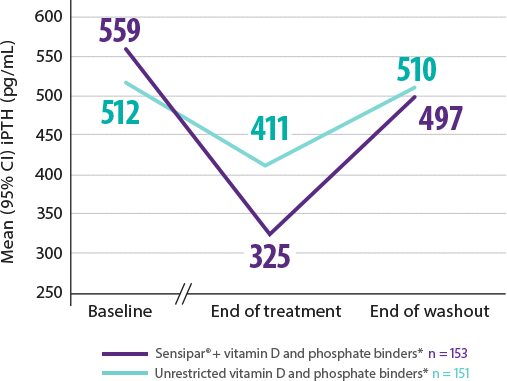
Adynamic Bone: Adynamic bone disease may develop if intact parathyroid hormone (iPTH) levels are suppressed below 100 pg/mL.
Adverse Reactions: In clinical trials of patients with secondary HPT comparing Sensipar® to placebo, the most commonly reported side effects were nausea (31% vs. 19%), vomiting (27% vs. 15%), and diarrhea (21% vs. 20%).
In clinical trials of patients with primary HPT and parathyroid carcinoma treated with Sensipar®, the most commonly reported side effects were nausea (63%), vomiting (46%), and paresthesia (20%)
Sensipar® (cinacalcet) is indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis.
Sensipar® (cinacalcet) is indicated for the treatment of hypercalcemia in adult patients with primary hyperparathyroidism (HPT) for whom parathyroidectomy would be indicated on the basis of serum calcium levels, but who are unable to undergo parathyroidectomy.
Sensipar® (cinacalcet) is indicated for the treatment of hypercalcemia in adult patients with parathyroid carcinoma.